A data fabric can break down data silos to help improve safety analyses, enabling more refined signal detection, benefit-risk profiles, and risk management action plans.
The value of big data is game-changing for life sciences firms equipped to leverage it. Life sciences firms have access to more data than ever, including internal and external data sources and real-world data. It can be difficult and time-consuming for them to bring together healthcare data across unstructured formats and multiple data sources, curate the data so it can be mined for insights, and empower users to draw and action the insights in real time. A data fabric can help.
Why? Firms that overcome these potential hurdles, however, can see massive gains. By seamlessly stitching together data, curating it for their needs, and enabling the smooth flow of data into actionable intelligence, it becomes possible to turn safety into a strategic center of value. Insights gained can help safety teams across several areas, including driving more efficient case processing, refining benefit-risk analyses, and ensuring greater patient safety.
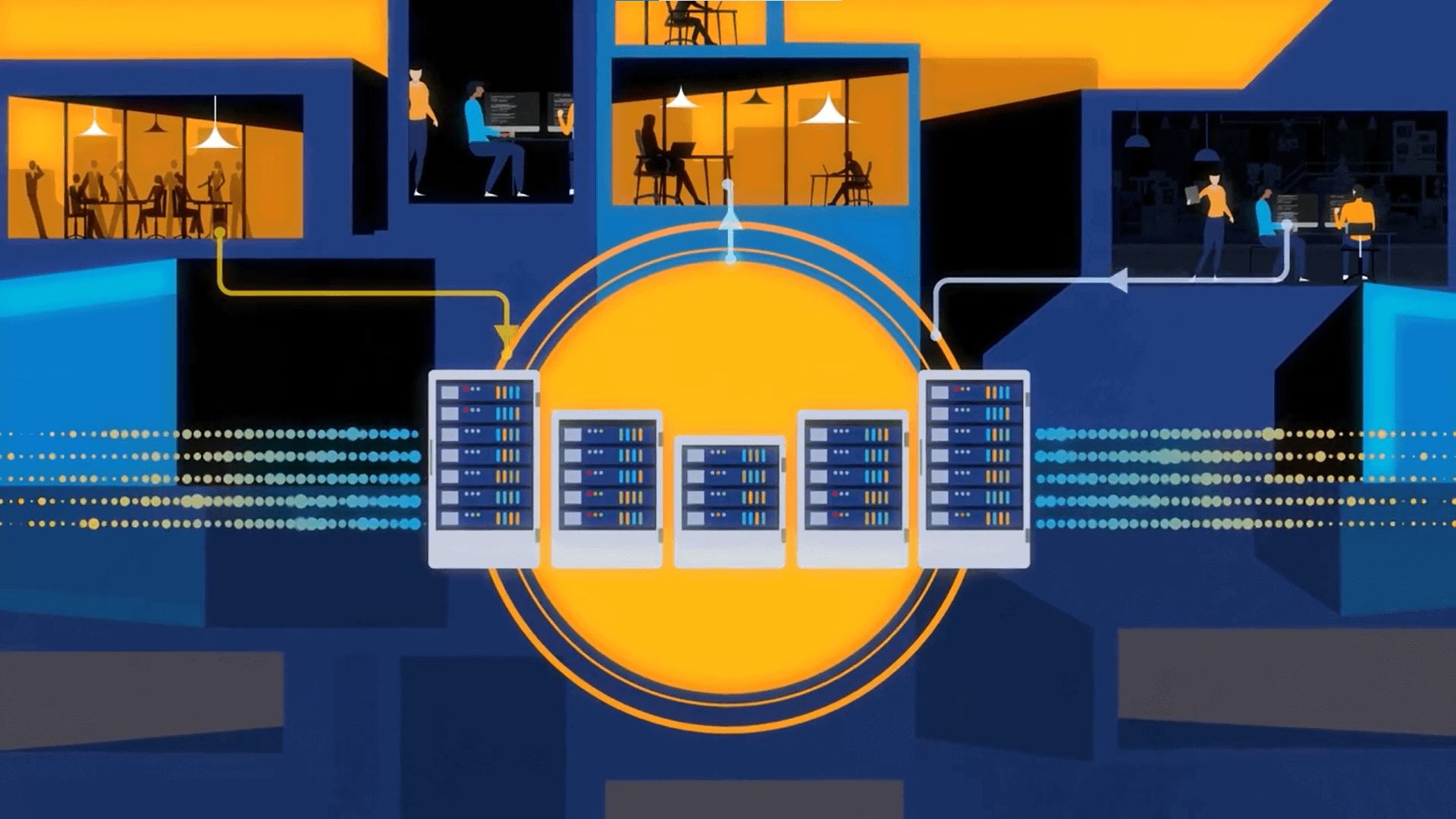
One major way firms are realizing this big data potential is by establishing a data fabric. A data fabric is an underlying architecture that helps firms more seamlessly stitch together data sources, support its consolidation and analysis, and make those insights actionable and accessible in real time. By leveraging this approach, firms can overcome siloes, noise, and friction to ensure they get the most out of all the data available to them.
Below, we touch more on the changing landscape of big data and how advancements in data and analytics can help turn these trends into opportunities.
See also: Business Leaders Must Make Data Fabrics a Priority in 2022
Managing higher case volumes and regulatory complexity
Safety case volumes continue to rise 30% to 50% annually due to factors such as the aging population, increasingly complex products and therapies, the rise in chronic diseases, and more sources available, including real-world data. Without ways to maximize processing efficiency as case volumes, safety teams incur added time and resource costs and have a harder time leveraging growing data for strategic insights.
Additionally, compliance regulations are only becoming more complex over time, driven by factors including the rise of new data sources, increasingly complex, targeted, and innovative personalized therapies, and a lack of harmonization across global regulators. Lacking the ability to reliably standardize and ensure up-to-date compliance across case intake, processing, and reporting can lead to added time and costs needed to remain compliant.
Leveraging a data fabric architecture can help break down silos and standardize case processing and compliance efficiencies across your operations. This includes streamlining key case intake, processing, and reporting workflows with pre-validated and tested AI, RPA, and rule-based automation to not only reduce PV cost burden and free resources to perform value-adding activities but they can leverage their data in a more strategic way, to drive greater operational efficiencies and patient safety.
Better leveraging real-world data
Real-world data (RWD) is one particularly critical source of data for firms to leverage. RWD is gathered from routine patient healthcare statuses, like electronic health records, and is studied to ensure patient safety. Sifting through these large quantities of information can help firms identify emerging risks and benefits and increase the robustness of a treatment’s safety profile.
Gartner suggests that about 80% of top life science companies use RWD to support R&D activities. So unsurprising is that regulators, specifically the U.S. Food and Drug Administration and the European Medicines Agency, have shown increased interest in studying and providing guidance on RWD. For example, the Big Data Task Force of the EMA and the Heads of Medicines Agencies notably said that the ability to use RWD analysis across multiple data sources contributes to the robustness of the evidence.
Establishing a data fabric architecture is one way to ensure your firm can not only bring in RWD to safety analyses but process it in a way that enables it to inform more refined signal detection, benefit-risk profiles, and risk management action plans.
Closing the gap between siloes
There is an increasing need to break silos for interoperability. Oftentimes, there are a high number of fragmented solutions that comprise a safety ecosystem – a number which is only growing as new data sources are introduced, and technology stacks become more complex. These siloes can not only make it difficult to conduct efficient PV operations and keep costs down, but they prevent you from making your safety data actionable.
The key to breaking these siloes is establishing end-to-end PV workflows. People, processes, and data should all be interconnected and need to be able to speak with one another. Architecture like the data fabric ensures this by stitching together your entire safety ecosystem and enabling data to be most valuable by enabling it to be seamlessly integrated and managed across different domains and readily actionable to stakeholders.
Ensuring patient safety
Of course, at the heart of pharmacovigilance is our commitment to maximizing patient safety.
As case volumes rise, regulations become more complex, and the ecosystem of data sources, teams, and processes grows, the need for a data architecture like data fabric is enabling firms to not only maintain efficient compliance throughout these trends but to unlock the next level of patient safety that is possible.
By seamlessly stitching data together, curating and analyzing it, and facilitating actionable insights, it becomes possible to detect deeper signals earlier in the safety lifecycle, use them to inform more robust benefit-risk profiles and go beyond compliance needs to proactively ensure patient safety. By being able to leverage new data sources like real-world data, more advanced automation/AI to analyze the data, and breaking down friction to accelerate insights into actionable steps, there is an exciting new opportunity to keep patients even safer.
Organizations, regulators, and providers should come together and collaborate, and technology should aid in maximizing patient outcomes. The impact of life sciences will only get stronger as technology continues to evolve and drive the safe development of therapies.